8. Elimination Reactions
Cumulative Substitution/Elimination
8. Elimination Reactions
Cumulative Substitution/Elimination - Video Tutorials & Practice Problems
On a tight schedule?
Get a 10 bullets summary of the topicTime to test yourself on what we've learned thus far. You are on your own here. We will be predicting mechanisms so keep the flowchart handy. Good luck!
1
concept
Intro to Substitution/Elimination Problems
Video duration:
38sPlay a video:
Time for some practice questions. Have a game plan ready and take it step by step. I believe in you all! Let's begin.
2
Problem
ProblemPredict the mechanism for the following reactions. Provide the full mechanism and draw the final product
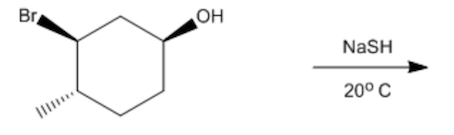
Video duration:
4mPlay a video:
Was this helpful?
3
Problem
Problem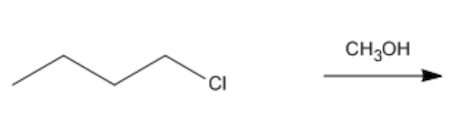
Video duration:
1mPlay a video:
Was this helpful?
4
Problem
Problem
Video duration:
4mPlay a video:
Was this helpful?
5
Problem
ProblemWhat is the major product for reaction d ?
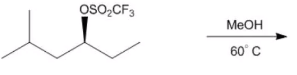
A
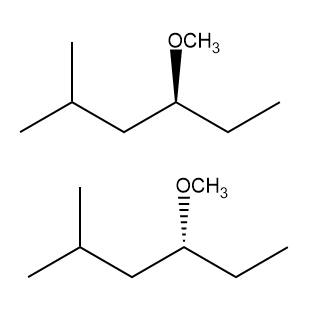
B
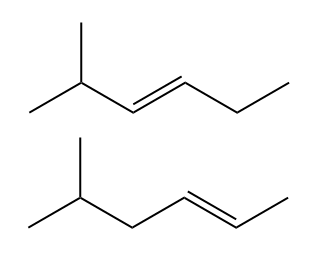
C
A and B
D
None of these
6
Problem
Problem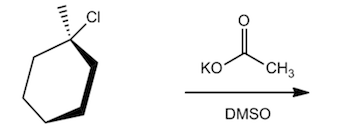
Video duration:
6mPlay a video:
Was this helpful?
7
Problem
Problem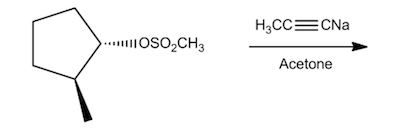
Video duration:
4mPlay a video:
Was this helpful?
Good job! Way to stick with it.
Do you want more practice?
We have more practice problems on Cumulative Substitution/Elimination
Additional resources for Cumulative Substitution/Elimination
PRACTICE PROBLEMS AND ACTIVITIES (30)
- What halides would undergo E2 dehydrohalogenation to give the following pure alkenes? a. Hex-1-ene b. Isobu...
- When the following compound is treated with sodium methoxide in methanol, two elimination products are possibl...
- Protonation converts the hydroxy group of an alcohol to a good leaving group. Suggest a mechanism for each rea...
- Draw the products of the following intramolecular reactions: a. b.
- After a proton is removed from the OH group, which compound in each pair forms a cyclic ether more rapidly? a...
- cis-4-Bromocyclohexanol and trans-4-bromocyclohexanol form the same elimination product but a different substi...
- Explain how each of the following changes affect the rate of the reaction of 1-bromobutane with ethoxide ion i...
- Explain how the following changes affect the rate of the reaction of 2-bromo-2-methylbutane with methanol: a....
- (••••) The following substitution reaction, between a strong base and a 1° haloalkane, occurs in a single step...
- Propose a mechanism for each of the following reactions: a.
- Which of the following two compounds eliminates HBr more rapidly in a basic solution?
- Propose a mechanism for the following reaction. (Hint: The rate of the reaction is much slower if the nitrogen...
- Show a mechanism for the following elimination reactions. Label the mechanism as E1 or E2.(d) <IMAGE>
- Paying close attention to the stereochemical outcome, predict the product of these elimination reactions.(d) ...
- (••) Predict the product(s) that would result when molecules (a)–(p) are allowed to react under the following ...
- (••) Predict the product(s) that would result when molecules (a)–(p) are allowed to react under the following ...
- (••) Predict the product(s) that would result when molecules (a)–(p) are allowed to react under the following ...
- (••) Predict the product(s) that would result when molecules (a)–(p) are allowed to react under the following ...
- (••) Predict the product(s) that would result when molecules (a)–(p) are allowed to react under the following ...
- (••) Predict the product(s) that would result when molecules (a)–(p) are allowed to react under the following ...
- (••) Predict the product(s) that would result when molecules (a)–(p) are allowed to react under the following ...
- (••) Predict the product(s) that would result when molecules (a)–(p) are allowed to react under the following ...
- (•••) Predict the product(s) of the following substitution or elimination reactions, paying close attention to...
- (•••) Predict the product(s) of the following substitution or elimination reactions, paying close attention to...
- (•••) Predict the product(s) of the following substitution or elimination reactions, paying close attention to...
- Show a mechanism for the following elimination reactions. Label the mechanism as E1 or E2.(c) <IMAGE>
- Would you expect the following bases to favor E1 or E2 elimination?(d) <IMAGE>
- Paying close attention to the stereochemical outcome, predict the product of these elimination reactions.(c) &...
- Indicate the type of catalysis that is occurring in the slow step in each of the following reaction sequences:...
- Indicate the type of catalysis that is occurring in the slow step in each of the following reaction sequences:...

