13. Alcohols and Carbonyl Compounds
Organometallic Cumulative Practice
13. Alcohols and Carbonyl Compounds
Organometallic Cumulative Practice - Video Tutorials & Practice Problems
On a tight schedule?
Get a 10 bullets summary of the topic1
concept
Intro to Predict the Product
Video duration:
24sPlay a video:
2
Problem
ProblemPredict the product of the reaction

A
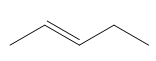
B
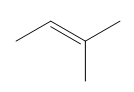
C
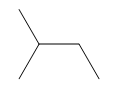
D
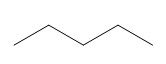
3
Problem
ProblemPredict the product of the reaction

A

B
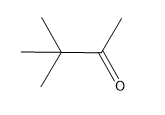
C

D

4
Problem
ProblemPredict the product of the reaction

A
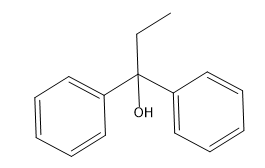
B

C
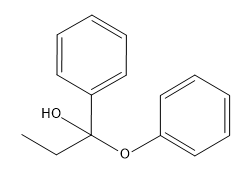
D
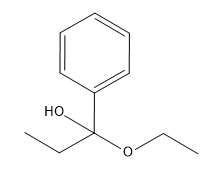
5
Problem
ProblemPredict the product of the reaction
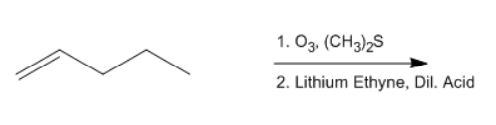
A

B
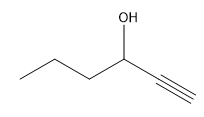
C
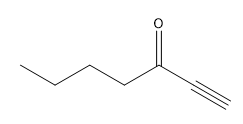
D

6
concept
Intro to Retrosynthesis
Video duration:
22sPlay a video:
7
Problem
ProblemPropose a synthesis to accomplish the following transformation
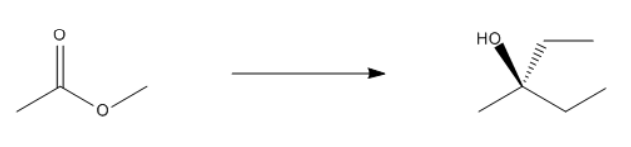
A
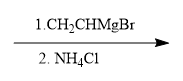
B
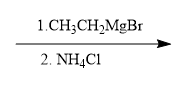
C

D
8
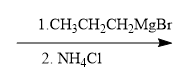
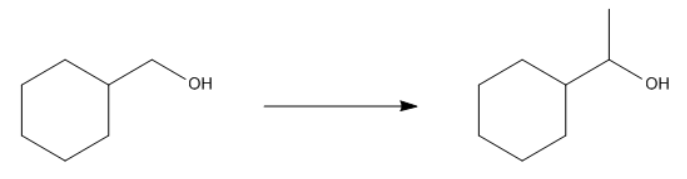
Problem
ProblemPropose a synthesis to accomplish the following transformation
A

B

C
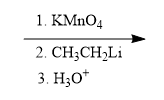
D

9
Problem
ProblemPredict the product of the reaction

A
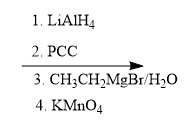
B

C

D

Do you want more practice?
We have more practice problems on Organometallic Cumulative Practice
Additional resources for Organometallic Cumulative Practice
PRACTICE PROBLEMS AND ACTIVITIES (15)
- Predict the product of the diorganocuprate cross-coupling reactions shown.(c) <IMAGE>
- Suggest a synthesis of the following molecule starting with the reagents shown, using cuprate cross-coupling a...
- (•••) Suggest a synthetic scheme, involving a protecting group, to generate the molecule shown starting with t...
- (•••) Suggest a synthetic scheme, involving a protecting group, to generate the molecule shown starting with t...
- Predict the product of the following reactions.(b) <IMAGE>
- Suggest a series of steps involving a cuprate reagent that would convert the reactant on the left to the produ...
- Suggest a reagent and a reactant that could be used to form the following molecules by conjugate addition of a...
- To this point, we have seen four reactions that can be done by Gilman reagents. What are they? What do they ha...
- (••) Phenol oxidation can be coupled with other reactions to form new C―C bonds using reactions studied previo...
- How could the following compounds be prepared, using cyclohexene as a starting material?a. <IMAGE>
- Using the given starting material, any necessary inorganic reagents and catalysts, and any carbon-containing c...
- Using cyclohexanone as the starting material, describe how each of the following compounds can be synthesized:...
- What are the products of the following reactions?b. <IMAGE>
- What are the products of the following reactions? Show all stereoisomers that are formed.a. <IMAGE>b. &l...
- What alcohols are formed from the reaction of ethylene oxide with the following organocuprates followed by the...

