10. Addition Reactions
Halohydrin
10. Addition Reactions
Halohydrin - Video Tutorials & Practice Problems
On a tight schedule?
Get a 10 bullets summary of the topicThis is an indentical mechanism to halogention, except with water as the nucleophile in the second step. Why would water prefer to react as a nucleophile over a halogen anion? Let's find out.
1
concept
General properties of halohydrin formation.
Video duration:
1mPlay a video:
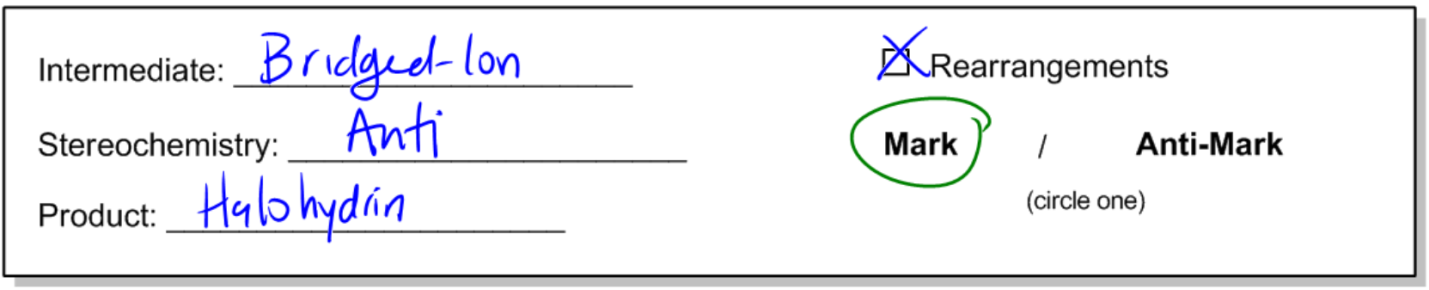
- Opening of 3-membered intermediates/molecules always results in anti-addition.
General Reaction:
2
concept
Halohydrin Mechanism
Video duration:
4mPlay a video:
1. Electrophilic Addition

2. Nucleophilic Substitution (SN2) and Deprotonation

3
Problem
ProblemPredict the product of the following reaciton.

A

B

C

D

4
Problem
ProblemPredict the product of the following reaction.
A
B
C
D
Do you want more practice?
We have more practice problems on Halohydrin
Additional resources for Halohydrin
PRACTICE PROBLEMS AND ACTIVITIES (38)
- What is the major product of each of the following reactions? b.
- What is the major product of each of the following reactions? b.
- What is the major product of each of the following reactions? a.
- What is the major product of each of the following reactions? e.
- Which stereoisomer of 3-hexene forms (3S,4S)-4-bromo-3-hexanol and (3R,4R)-4-bromo-3-hexanol when it reacts wi...
- Predict the major product(s) for each reaction. Include stereochemistry where appropriate. c. cis-but-2-ene +...
- Predict the major product(s) for each reaction. Include stereochemistry where appropriate. a. 1-methylcyclohe...
- Propose a mechanism for the addition of bromine water to cyclopentene, being careful to show why the trans pro...
- Show how you would make the following compounds from a suitable cyclic alkene. d.
- Show how you would accomplish the following synthetic conversions. a. 3-methylpent-2-ene--> 2-chloro-3-met...
- b. Predict the product of formula C7H13O from the reaction of this same unsaturated alcohol with bromine. Prop...
- Suggest an alkene that could be used to make each of the following halohydrins. (a)
- Predict the product(s) of each of the following reactions, making sure to indicate the relative stereochemical...
- Provide arrow-pushing mechanisms for Assessments 9.10(b) and 9.10(c) that rationalize the regioselective and s...
- (••) Predict the product(s) that would result when the alkenes are allowed to react under the following condit...
- (••) Predict the product(s) that would result when the alkenes are allowed to react under the following condit...
- (••) Predict the product(s) that would result when the alkenes are allowed to react under the following condit...
- (••) Predict the product(s) that would result when the alkenes are allowed to react under the following condit...
- (••) Predict the product(s) that would result when the alkenes are allowed to react under the following condit...
- (••) Predict the product(s) that would result when the alkenes are allowed to react under the following condit...
- (••) At the beginning of Chapter 9, we stated that after finishing Chapters 8 and 9, we would have the ability...
- (••) When alkenes react with bromine in water, a halohydrin is produced. When water is replaced with methanol ...
- Explain why water attacks the carbon of the bromonium ion as opposed to the bromonium ion itself in the second...
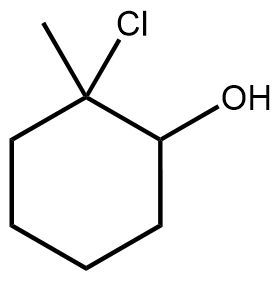
- Suggest an alkene that could be used to make each of the following halohydrins.(c) <IMAGE>
- (••) Predict the product(s) that would result when the alkenes are allowed to react under the following condit...
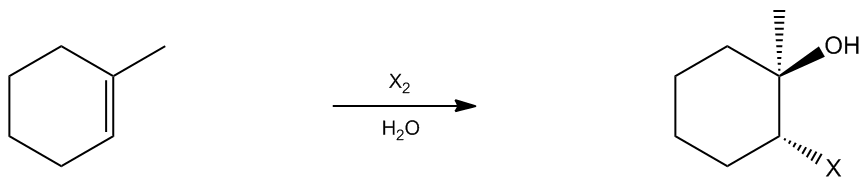
- Predict the product of the following haloalkane syntheses.(e) ↓ Cl₂₊ <IMAGE> H₂O
- Halohydrin formation is a stereospecific reaction. Identify the products of halohydrin formation of the follow...
- (•••) In light of your answer to Assessment 9.47, predict the product of the following reactions we have seen ...
- (••) Predict the product(s) that would result when the alkenes are allowed to react under the following condit...
- (••) Predict the product(s) that would result when the alkenes are allowed to react under the following condit...
- (•••) Retrosynthetic analysis is the process of working backward to develop the synthesis of a new compound. I...
- What will be the major product obtained from the reaction of Br2 with 1-butene if the reaction is carried out ...
- 1-Methylcyclohexene forms two products when it reacts with bromine in methanol.a. Draw the mechanism for the f...
- Using any alkene and any other reagents, how would you prepare the following compounds?d. <IMAGE>
- What will be the major product obtained from the reaction of Br2 with 1-butene if the reaction is carried out ...
- Draw the mechanism for the following reaction:<IMAGE>
- Each of the following reactions has two nucleophiles that could add to the intermediate formed by the reaction...
- What is the major product of the reaction of 2-methyl-2-butene with each of the following reagents?j. Br2 >...