8. Elimination Reactions
E2 - Cumulative Practice
8. Elimination Reactions
E2 - Cumulative Practice - Video Tutorials & Practice Problems
On a tight schedule?
Get a 10 bullets summary of the topicWe just tackled the anti-coplanar requirement for E2 reactions. Now, let's see if you can draw the correct final products using the same molecules we've seen in the past. Good luck!
1
concept
E2 Cumulative Intro
Video duration:
27sPlay a video:
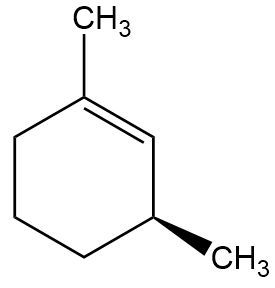
A. Provide the full mechanism and draw the final product for the following E2 reaction.
2
Problem
Problem
Video duration:
1mPlay a video:
Was this helpful?
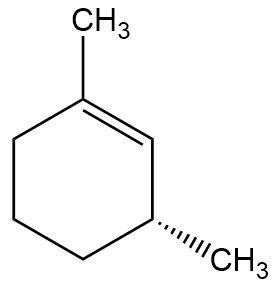
B. Provide the full mechanism and draw the final product for the following E2 reaction.
3
Problem
Problem
Video duration:
1mPlay a video:
Was this helpful?
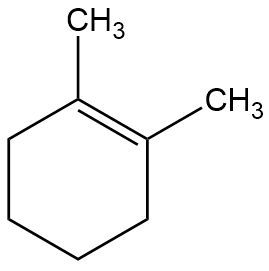
C. Provide the full mechanism and draw the final product for the following E2 reaction.
4
Problem
Problem
A
B
C
D
Great job guys.:)
Keep up the awesome work and you will be pro's at the E2 reaction in no time.
Do you want more practice?
We have more practice problems on E2 - Cumulative Practice