22. Condensation Chemistry
Condensation Reactions
22. Condensation Chemistry
Condensation Reactions - Video Tutorials & Practice Problems
On a tight schedule?
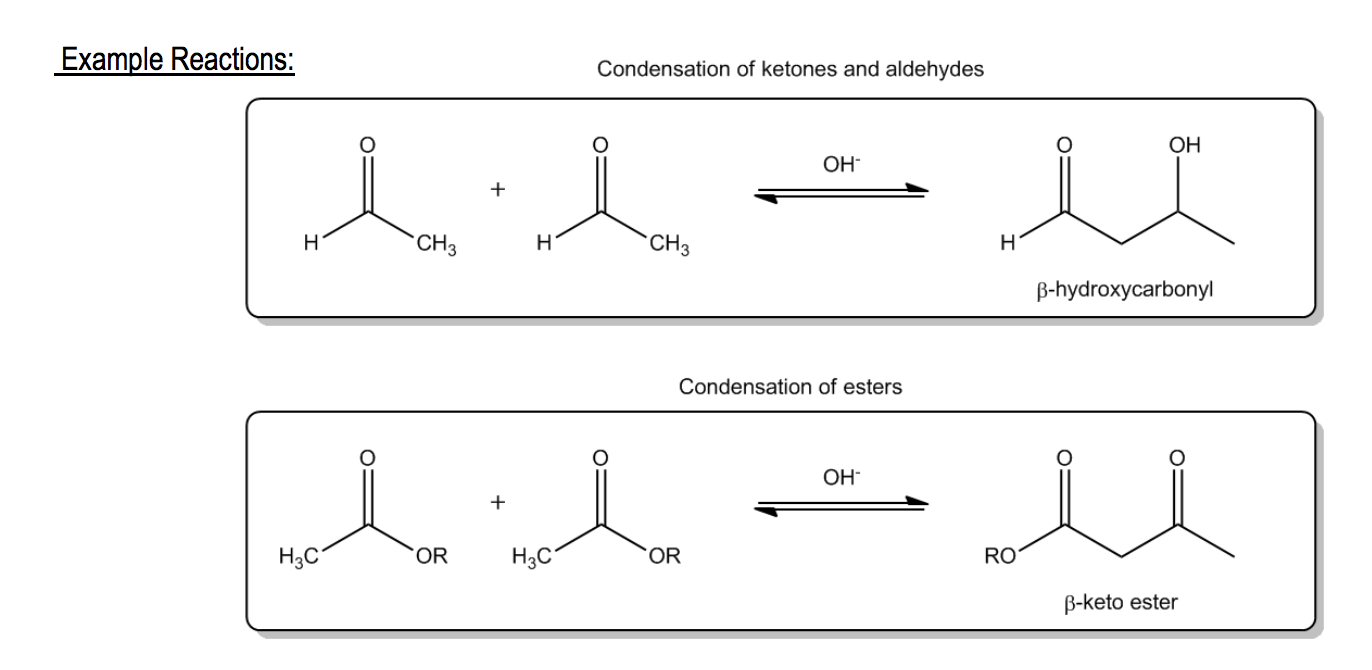
Get a 10 bullets summary of the topicCondensation occurs when 2 molecules spontaneously combine to form a larger molecule with the loss of a smaller molecule.
Let's take a look now at how enolates come into the picture.
1
concept
Condensation Reactions
Video duration:
5mPlay a video:
Do you want more practice?
We have more practice problems on Condensation Reactions
Additional resources for Condensation Reactions
PRACTICE PROBLEMS AND ACTIVITIES (45)
- What products would you expect to obtain from the following reactions? b. methyl carbamate + methylamine
- What products would you expect to obtain from the following reactions? a. malonic acid+2 acetyl chloride
- What compounds are obtained from the following hydrolysis reactions? d. CH3CH2C=O(O)C=O(CH3) + H2O —>
- What compounds are formed from the reaction of benzoyl chloride with the following reagents? k. potassium for...
- What compounds are formed from the reaction of benzoyl chloride with the following reagents? i. isopropyl alc...
- What compounds are formed from the reaction of benzoyl chloride with the following reagents? f. cyclohexanol
- What compounds are formed from the reaction of benzoyl chloride with the following reagents? e. aqueous NaOH
- b. How does acetic anhydride make it easier to form the anhydride?
- a. Propose a mechanism for the formation of succinic anhydride from succinic acid in the presence of acetic an...
- We saw that acid anhydrides react with alcohols, water, and amines. In which of these reactions can the tetrah...
- a. Propose a mechanism for the reaction of acetic anhydride with water.
- Write the mechanism for the acid-catalyzed reaction of an amide with an alcohol to form an ester.
- Write a mechanism for each of the following reactions: b. the aminolysis of phenyl formate, using methylamine...
- Write a mechanism for each of the following reactions: a. the uncatalyzed hydrolysis of methyl propionate.
- Starting with acetyl chloride, what neutral nucleophile would you use to synthesize each of the following comp...
- Starting with acetyl chloride, what neutral nucleophile would you use to synthesize each of the following comp...
- Starting with acetyl chloride, what neutral nucleophile would you use to synthesize each of the following comp...
- Starting with acetyl chloride, what neutral nucleophile would you use to synthesize each of the following comp...
- Using the pKa values listed in [TABLE 15.1] , predict the products of the following reactions: d. + NaOH —&g...
- Using the pKa values listed in [TABLE 15.1] , predict the products of the following reactions: c. + NaCl —&g...
- What is the product of an acyl substitution reaction—a new carboxylic acid derivative, a mixture of two carbo...
- What is the product of an acyl substitution reaction—a new carboxylic acid derivative, a mixture of two carbo...
- b. What is the product of the reaction of acetamide with HO−? The pKa of NH3 is 36; the pKa of H2O is 15.7.
- Which of the following reactions lead to the formation of an amide? A. RC=O(OH) + CH3NH2 —> B. RC=O(OCH3)...
- What compounds are obtained from the following hydrolysis reactions? c. + H2O HCl Δ—>
- Write the mechanism for the acid-catalyzed reaction of tert-butyl acetate with methanol.
- Catalytic antibodies catalyze a reaction by forcing the conformation of the substrate in the direction of the ...
- The following compound has been found to be an inhibitor of penicillinase. The enzyme can be reactivated by hy...
- The following compound has been found to be an inhibitor of penicillinase. The enzyme can be reactivated by hy...
- Propose a mechanism for each of the following reactions: b.
- Draw the products of the following reactions: d.
- Draw the products of the following reactions: c.
- How could you prepare the following compound using a starting material that contains no more than three carbon...
- There are other condensation reactions similar to the aldol and Claisen condensations: a. The Perkin condensat...
- Identify A–J:
- Show how the following compounds can be prepared from the given starting materials. You can use any necessary ...
- Refer to Figure 20.5 to answer the following questions: b. People with type AB blood can receive blood from a...
- Refer to Figure 20.5 to answer the following questions: a. People with type O blood can donate blood to anyone...
- Draw the product obtained when a lysine side chain in a polypeptide reacts with maleic anhydride.
- What dipeptides would be formed by heating a mixture of valine and N-protected leucine?
- Propose a mechanism for the rearrangement of the thiazoline obtained from the reaction of Edman's reagent with...
- Proof that an imine was formed between aldolase and its substrate was obtained by using d-fructose-1,6-bisphos...
- Which compound forms an anhydride more rapidly?
- Why do the nitro groups change the relative leaving tendencies of the carboxy and 2,4-dinitrophenoxy groups in...
- Co2 + catalyzes the hydrolysis of the lactam shown here. Propose a mechanism for the metal-ion catalyzed react...

