4. Alkanes and Cycloalkanes
Cis vs Trans Conformations
4. Alkanes and Cycloalkanes
Cis vs Trans Conformations - Video Tutorials & Practice Problems
On a tight schedule?
Get a 10 bullets summary of the topicBefore we can really understand chair conformations, we have to practice drawing them! These tricky little suckers can be hard to get right.
Method for Drawing Chairs
1
concept
How to draw chairs.
Video duration:
1mPlay a video:
- Draw two slightly angled parallel lines.

- Cap both ends off

How did you do?
It's ok if you didn't draw it beautifully the first time - practice makes perfect!
Cis and Trans
2
concept
How to distinguish cis from trans.
Video duration:
39sPlay a video:
Cis or trans is based on whether the groups are facing the same face (top or bottom) of the ring.
- It has nothing to do with the axial and equatorial positions!
3
example
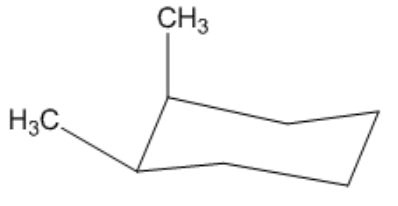
Is the following cyclohexane cis or trans?
Video duration:
44sPlay a video:
4
example
Is the following cyclohexane cis or trans?
Video duration:
27sPlay a video:
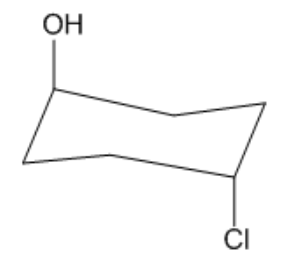
Not too bad, right? It's as easy as it sounds! Let's move on.
Do you want more practice?
We have more practice problems on Cis vs Trans Conformations
Additional resources for Cis vs Trans Conformations
PRACTICE PROBLEMS AND ACTIVITIES (14)
- Name the following compounds. Remember that two up bonds are cis; two down bonds are cis; one up bond and one ...
- Which of the following structures represent the same compound? Which ones represent different compounds? e. &...
- Given the following chair conformations, draw each in its planar form as if you were viewing it from above. (...
- Given the following chair conformations, draw each in its planar form as if you were viewing it from above. (...
- Is each of the following a cis isomer or a trans isomer? a. b. c.
- For each of the following compounds, determine whether the cis isomer or the trans isomer is more stable.
- Given the following chair conformations, draw each in its planar form as if you were viewing it from above. (...
- Name the following compounds. Remember that two up bonds are cis; two down bonds are cis; one up bond and one ...
- Use your results from [PROBLEM 3-27] to complete the following table . Each entry shows the positions of two g...
- For each of the following compounds, draw the possible geometric isomers and name each isomer: a. 2-methyl-2,4...
- For each of the following compounds, draw the possible geometric isomers and name each isomer: b. 1,5-heptadie...
- For the compound 1-ethyl-3-isopropylcyclopentane,(c) What is the stereochemical relationship between trans iso...
- Draw two different chair conformations for each of the following molecules. Make sure that your drawings clear...
- Which of the following represents a cis isomer?<IMAGE>