1. Matter and Measurements
Significant Figures: Precision in Measurements
1. Matter and Measurements
Significant Figures: Precision in Measurements - Video Tutorials & Practice Problems
On a tight schedule?
Get a 10 bullets summary of the topicSignificant Figures are used to discuss the level of precision in any measurement.
Recording Measurements
1
concept
Significant Figures Precision Concept
Video duration:
1mPlay a video:
2
example
Significant Figures Precision Example
Video duration:
1mPlay a video:
3
Problem
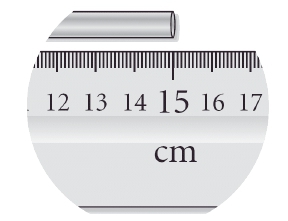
ProblemRead the length of the metal bar to the correct number of significant figures.
A
15 cm
B
15.000 cm
C
20 cm
D
15.0 cm
E
15.00 cm
4
Problem
ProblemWhat is the correct reading for the liquid in the burette provided below?
A
32 mL
B
32.30 mL
C
32.26 mL
D
32.0 mL
E
32.2 mL
Do you want more practice?
We have more practice problems on Significant Figures: Precision in Measurements
Additional resources for Significant Figures: Precision in Measurements
PRACTICE PROBLEMS AND ACTIVITIES (5)
- Use the following graph for problems1.23 and 1.24: d. How many minutes were needed to reach a temperature of...
- a. What is the specific gravity of the following solution?
- Assume that you are delivering a solution sample from a pipette. Figures (a) and (b) show the volume level bef...
- Measure the length of each of the objects in diagrams (a), (b), and (c) using the metric ruler in the figure. ...
- State the temperature on the Celsius thermometer to the correct number of significant figures: (2.3) A