10. Periodic Properties of the Elements
Paramagnetism and Diamagnetism
10. Periodic Properties of the Elements
Paramagnetism and Diamagnetism - Video Tutorials & Practice Problems

Get help from an AI Tutor
Ask a question to get started.
Paramagnetism and Diamagnetism deal with the presence of electrons that are either unpaired or paired in an orbital.
Paramagnetism & Diamagnetism
1
concept
Paramagnetism and Diamagnetism
Video duration:
1mPlay a video:
A paramagnetic element has at least one unpaired electron and a diamagnetic element has no unpaired electrons.
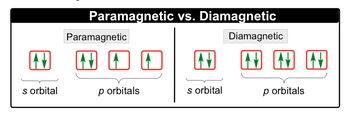
2
example
Paramagnetism and Diamagnetism Example 1
Video duration:
1mPlay a video:
3
Problem
ProblemWhich of the following atoms has the most unpaired electrons?
A
Co
B
Mn
C
Ti
D
Zn
E
Fe
4
Problem
ProblemWrite the condensed electron configuration for the nickel (III) ion and state if it is paramagnetic or diamagnetic.
Video duration:
2mPlay a video:
Was this helpful?
5
Problem
ProblemWrite the condensed electron configuration for the copper (I) ion and is it magnetic?
A
[Ar]4s23d9 ; Diamagnetic
B
; Diamagnetic
C
[Ar]4s23d9 ; Paramagnetic
D
[Ar]3d10 ; Paramagnetic
Do you want more practice?
We have more practice problems on Paramagnetism and Diamagnetism
Additional resources for Paramagnetism and Diamagnetism
PRACTICE PROBLEMS AND ACTIVITIES (25)
- State where in the periodic table these elements appear: (a) elements with the valence-shell electron configu...
- Write orbital diagrams for each of these ions.Determine if the ion is diamagnetic or paramagnetic. a. V5+ b....
- Write orbital diagrams for each ion and determine if the ion is diamagnetic or paramagnetic. a. Cd2+ b. Au+ ...
- An experiment called the Stern–Gerlach experiment helped establish the existence of electron spin. In this ex...
- Write the condensed electron configurations for the following atoms and indicate how many unpaired electrons e...
- Write the condensed electron configurations for the following atoms and indicate how many unpaired electrons ...
- Identify the specific element that corresponds to each of the following electron configurations and indicate ...
- Identify the specific element that corresponds to each of the following electron configurations and indicate ...
- Identify the specific element that corresponds to each of the following electron configurations and indicate t...
- (a) What does the term paramagnetism mean? (b) How can one determine experimentally whether a substance is pa...
- Both vanadium and its 3+ ion are paramagnetic. Refer to their electron configurations to explain this stateme...
- Using the periodic table as a guide, write the condensed electron configuration and determine the number of u...
- In the experiment shown schematically below, a beam of neutral atoms is passed through a magnetic field. Atom...
- In the experiment shown schematically below, a beam of neutral atoms is passed through a magnetic field. Atoms...
- Carbon monoxide is produced by incomplete combustion of fossil fuels. (b) Do you expect CO to be paramagnetic ...
- How many unpaired electrons are present in each of the following ground-state atoms? (a) O (b) Si (c) K (d)...
- Consider the elements: B, C, N, O, F. d. Which element has three unpaired electrons?
- Consider the elements: Na, Mg, Al, Si, P. d. Which element is diamagnetic?
- Cyclooctatetraene dianion, C8H8 2-, is an organic ion with the structure shown. Considering only the p bonds...
- In the ground-state electron configuration of Fe3+, how many unpaired electrons are present?
- Select the element(s) that will have one unpaired electron in the p orbital.
- Identify the general outer electron configuration for each group of elements shown in this periodic table outl...
- Sort the following atom or ions as paramagnetic or diamagnetic according to the electron configurations determ...
- In the ground-state electron configuration of Fe3+, how many unpaired electrons are present?
- How many unpaired electrons are present in the ground state of the atoms in group 6A(16)?

