13. Liquids, Solids & Intermolecular Forces
Face Centered Cubic Unit Cell
13. Liquids, Solids & Intermolecular Forces
Face Centered Cubic Unit Cell - Video Tutorials & Practice Problems

Get help from an AI Tutor
Ask a question to get started.
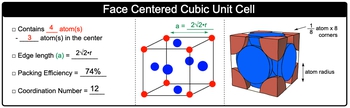
Face Centered Cubic Unit Cell contains 4 atoms in total.
Face Centered Cubic Unit Cell
1
concept
Face Centered Cubic Unit Cell Concept 1
Video duration:
1mPlay a video:
2
example
Face Centered Cubic Unit Cell Example 1
Video duration:
51sPlay a video:
3
Problem
ProblemAluminum has a face-centered cubic unit structure and a density of 2.716 g/cm3. Calculate the edge length of the unit cell.
A
4.041×10–8 cm
B
3.992×10–8 cm
C
3.615×10–8 cm
D
3.247×10–8 cm
E
2.836×10–8 cm
4
Problem
ProblemAn element crystallizes in a face-centered cubic lattice and has a density of 1.45 g/cm3. The edge of its unit cell is 4.52×10–8 cm. How many atoms are in each unit cell?
A
4 atoms
B
2 atoms
C
1 atom
D
3 atoms
5
Problem
ProblemAn element crystallizes in a face-centered cubic lattice and has a density of 18.44 g/cm3. The edge of its unit cell is 1.05×10-8 cm. Calculate the atomic mass for the element.
A
3.21 g•mol-1
B
15.5 g•mol-1
C
60.8 g•mol-1
D
151 g•mol-1
6
Problem
ProblemZinc selenide, ZnSe, crystallizes in a face-centered cubic unit cell has a density of 5.42 g/cm3. What is the volume of a unit cell?
A
7.17×10–24 cm3
B
1.19×10–11 cm3
C
8.03×105 cm3
D
1.77×10–22 cm3
Do you want more practice?
We have more practice problems on Face Centered Cubic Unit Cell
Additional resources for Face Centered Cubic Unit Cell
PRACTICE PROBLEMS AND ACTIVITIES (38)
- Niobium oxide crystallizes in the following cubic unit cell: What is the formula of niobium oxide, and what i...
- Titanium oxide crystallizes in the following cubic unit cell: (b) What is the formula of titanium oxide?
- With the exception of helium, the noble gases condense to form solids when they are cooled sufficiently. At t...
- What is the minimum number of atoms that could be contained in the unit cell of an element with a face-center...
- Determine the number of atoms per unit cell for each metal. (c) Nickel
- Consider the unit cells shown here for three different structures that are commonly observed for metallic elem...
- Platinum crystallizes with the face-centered cubic unit cell. The radius of a platinum atom is 139 pm. Calcula...
- Rhodium has a density of 12.41 g/cm3 and crystallizes with the face-centered cubic unit cell. Calculate the ra...
- Copper crystallizes in a face-centered cubic unit cell with an edge length of 362 pm. What is the radius of a...
- Aluminum has a density of 2.699 g>cm3 and crystallizes with a face-centered cubic unit cell. What is the e...
- An element crystallizes in a face-centered cubic lattice. The edge of the unit cell is 4.078 Å, and the densi...
- Titanium metal has a density of 4.506 g>cm3 and an atomic radius of 144.8 pm. In what cubic unit cell does...
- The atomic radius of Pb is 175 pm, and the density is 11.34 g>cm3. Does lead have a primitive cubic struct...
- The density of a sample of metal was measured to be 6.84 g>cm3. An X-ray diffraction experiment measures t...
- Sodium hydride, NaH, crystallizes in a face-centered cubic unit cell similar to that of NaCl (Figure 12.11). ...
- Identify the structure of each of the two unit cells shown in Problem 48 as the rock salt structure, zinc blen...
- If the edge length of an NaH unit cell is 488 pm, what is the length in picometers of an Na¬H bond? (See Prob...
- Tausonite, a mineral composed of Sr, O, and Ti, has the cubic unit cell shown in the drawing. (a) What is the ...
- A particular form of cinnabar (HgS) adopts the zinc blende structure. The length of the unit cell edge is 5.8...
- A particular form of cinnabar (HgS) adopts the zinc blende structure. The length of the unit cell edge is 5.85...
- CuI, CsI, and NaI each adopt a different type of structure. The three different structures are those shown in ...
- Copper iodide crystallizes in the zinc blende structure. The sep- aration between nearest neighbor cations and...
- The density of an unknown metal is 12.3 g/cm3, and its atomic radius is 0.134 nm. It has a face-centered cubic...
- The ionic compound CaO crystallizes with the same structure as sodium chloride (Figure 8.3). (a) In this struc...
- Calculate the fraction of empty space in cubic closest packing to five significant figures.
- The ionic substance strontium oxide, SrO, forms from the reaction of strontium metal with molecular oxygen. T...
- The ionic substance strontium oxide, SrO, forms from the reaction of strontium metal with molecular oxygen. T...
- Pure iron crystallizes in a body-centered cubic structure, shown in the figure. but small amounts of impuriti...
- For each of the intermetallic compounds shown in Figure 12.17 determine the number of each type of atom in the...
- What type of lattice—primitive cubic, body-centered cubic, or face-centered cubic—does each of the following ...
- Platinum nanoparticles of diameter 2 nm are important catalysts in carbon monoxide oxidation to carbon dioxide...
- Platinum nanoparticles of diameter 2 nm are important catalysts in carbon monoxide oxidation to carbon dioxide...
- Sodium oxide (Na2O) adopts a cubic structure with Na atoms represented by green spheres and O atoms by red sph...
- (a) The density of diamond is 3.5 g>cm3, and that of graphite is 2.3 g>cm3. Based on the structure of bu...
- Silicon has the diamond structure with a unit cell edge length of 5.43 Å and eight atoms per unit cell. (b) S...
- The mineral wustite is a nonstoichiometric iron oxide with the empirical formula FexO, where x is a number sli...
- The alkali metal fulleride superconductors M3C60 have a cubic closest-packed (face-centered cubic) arrangemen...
- Assuming that the ionic radius of oxygen is 140 pm, estimate the ionic radius of manganese.

