15. Chemical Kinetics
Average Rate of Reaction
15. Chemical Kinetics
Average Rate of Reaction - Video Tutorials & Practice Problems

Get help from an AI Tutor
Ask a question to get started.
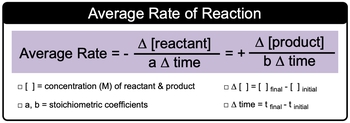
Average (General) Rate is the change in the concentration of a compound over a period of time.
Average Rate
1
concept
Average Rate of Reaction Concept 1
Video duration:
2mPlay a video:
2
example
Average Rate of Reaction Example 1
Video duration:
1mPlay a video:
3
example
Average Rate of Reaction Example 2
Video duration:
2mPlay a video:
4
Problem
ProblemConsider the following reaction:2 H2O2 (aq) → 2 H2O (l) + O2 (g)
Calculate the rate of the reaction between 25 sec and 65 sec.
A
0.018 M/s
B
9.0×10–3 M/s
C
0.036 M/s
D
0.041 M/s
E
0.083 M/s
Do you want more practice?
We have more practice problems on Average Rate of Reaction
Additional resources for Average Rate of Reaction
PRACTICE PROBLEMS AND ACTIVITIES (28)
- Use the following equation and graph to answer questions 1 and 2. Hydrogen iodide decomposes at 410 °C, accord...
- You study the rate of a reaction, measuring both the concentration of the reactant and the concentration of th...
- (a) What are the units usually used to express the rates of reactions occurring in solution?
- Consider the following hypothetical aqueous reaction: A1aq2S B1aq2. A flask is charged with 0.065 mol of A in ...
- Consider the following hypothetical aqueous reaction: A1aq2S B1aq2. A flask is charged with 0.065 mol of A in ...
- A flask is charged with 0.100 mol of A and allowed to react to form B according to the hypothetical gas-phase ...
- The isomerization of methyl isonitrile 1CH3NC2 to acetonitrile 1CH3CN2 was studied in the gas phase at 215 C, ...
- The rate of disappearance of HCl was measured for the following reaction: CH3OH1aq2 + HCl1aq2¡CH3Cl1aq2 + H2O1...
- The rate of disappearance of HCl was measured for the following reaction: CH3OH1aq2 + HCl1aq2¡CH3Cl1aq2 + H2O1...
- The rate of disappearance of HCl was measured for the following reaction: CH3OH1aq2 + HCl1aq2¡CH3Cl1aq2 + H2O1...
- For each of the following gas-phase reactions, indicate how the rate of disappearance of each reactant is rela...
- (b) The reaction 2 NO1g2 + Cl21g2¡2 NOCl1g2 is carried out in a closed vessel. If the partial pressure of NO i...
- Consider the reaction: 2 HBr( g) ¡ H2( g) + Br2( g) a. Express the rate of the reaction in terms of the chang...
- Consider the reaction:2 HBr (g) → H2 (g) + Br2 (g)c. If the volume of the reaction vessel in part b was 1.50 L...
- Consider the reaction: 2 HBr( g) ¡ H2( g) + Br2( g) b. In the first 25.0 s of this reaction, the concentratio...
- Consider the reaction: 2 N2O( g) ¡ 2 N2( g) + O2( g) a. Express the rate of the reaction in terms of the chang...
- For the reaction 2 A( g) + B( g) ¡ 3 C( g), b. when A is decreasing at a rate of 0.100 M>s, how fast is B d...
- For the reaction 2 A( g) + B( g) ¡ 3 C( g), a. determine the expression for the rate of the reaction in terms ...
- Consider the reaction: 8 H2S( g) + 4 O2( g) ¡ 8 H2O( g) + S8( g) Complete the table.
- Consider the reaction: C4H8( g) ¡ 2 C2H4( g) The tabulated data were collected for the concentration of C4H8 a...
- Consider the reaction: NO2( g) ¡ NO( g) + 1 2 O2( g) The tabulated data were collected for the concentration o...
- Consider the reaction: NO2( g) ¡ NO( g) + 1 2 O2( g) The tabulated data were collected for the concentration o...
- Consider the reaction: H2( g) + Br2( g) ¡ 2 HBr( g) The graph shows the concentration of Br2 as a function of ...
- Consider the reaction: 2 H2O2(aq) ¡ 2 H2O(l ) + O2( g) The graph shows the concentration of H2O2 as a function...
- A 1.50 L sample of gaseous HI having a density of 0.0101 g>cm3 is heated at 410 °C. As time passes, the H...
- Which species has the greatest rate of appearance in the reaction below? 2 H2S + O2 → 2 S + 2 H2O
- Which species has the greatest rate of disappearance in the reaction below? CH4 + 2 O2 → CO2 + 2 H2O
- Which species has the greatest rate of disappearance in the reaction below? CH4 + 2 O2 → CO2 + 2 H2O
